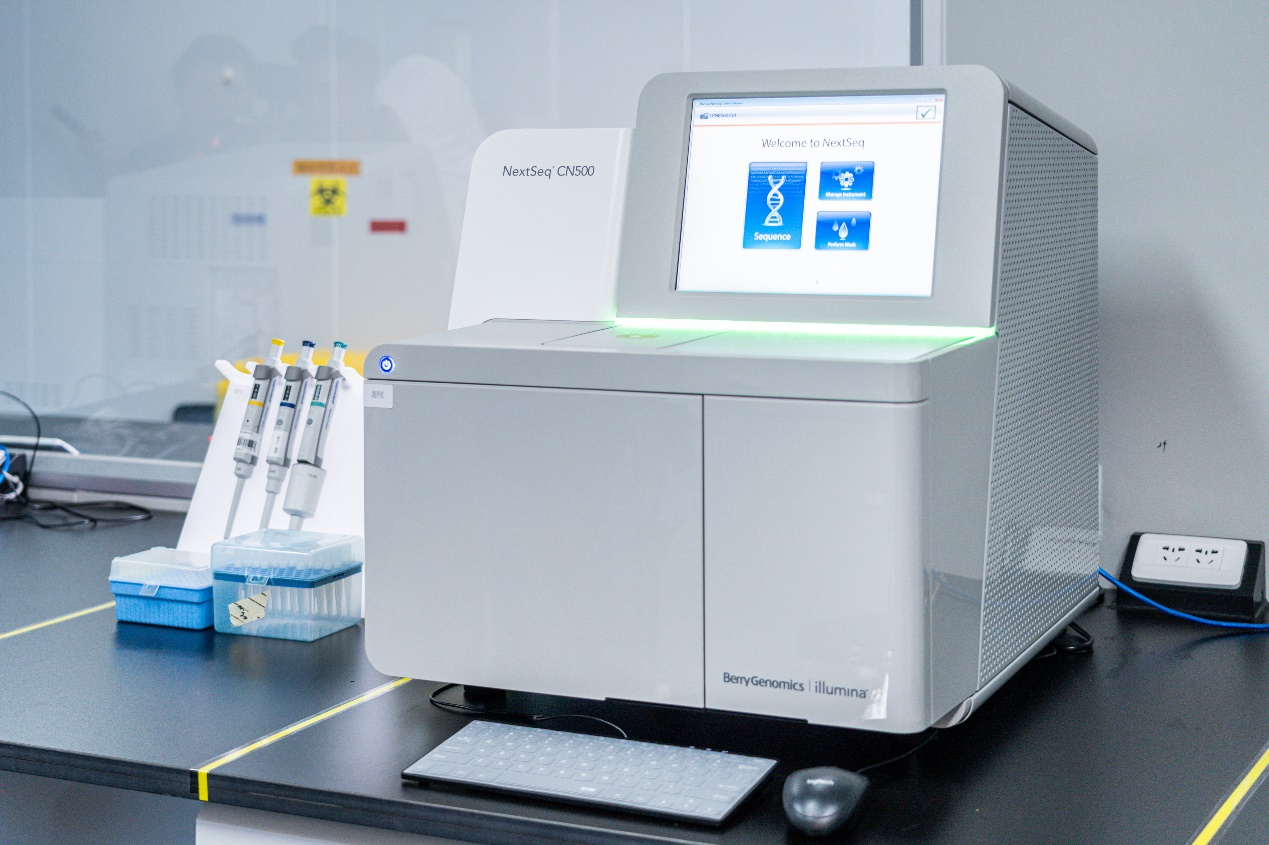
Nextseq cn500 gene sequencer (Registration Certificate No.: gxzz 20153220460) adopts a convenient icon touch operating system. It only takes 10 minutes to add samples and set the system before operation. After operation, there is no need for manual operation, personnel supervision and other specified supporting equipment. All sequencing reagents were prefabricated and ready to use. It can realize exome sequencing, whole genome sequencing and transcriptome sequencing.
At present, Nanxin medical laboratory mainly uses this instrument to detect clinical pathogenic macro gene and macro transcription, as well as sequencing related scientific research projects.
Technology Platform
Second Generation Sequencing Platform

MiSeq ™ DX is Illumina's first imported gene sequencer registered and approved by the State Drug Administration of China (Registration Certificate No.: gxzj 20183400291). Miseq DX of Nanxin medical laboratory is the first sequencer installed in South China. It is composed of miseq DX instrument and miseq DX software, which can be used for in vitro diagnosis.
At present, Nanxin medical laboratory has carried out 16S high-throughput sequencing of multi population, multi disease models and various types through the instrument, especially in the detection of human intestinal microflora. At present, it has established a variety of human micro ecological data groups.
At present, Nanxin medical laboratory has carried out 16S high-throughput sequencing of multi population, multi disease models and various types through the instrument, especially in the detection of human intestinal microflora. At present, it has established a variety of human micro ecological data groups.



 En
En